
Starting date :
Jan. 2024 > Dec. 2027
Lifetime :
48 months
Program in support :
Horizon Europe (HORIZON-CL4-2023-RESILIENCE-01-21)
Status project :
in progress

CEA-Leti's contact :
Project Coordinator :
Steinbeis 2i GmbH (DE)
Partners: - CEA-Leti (FR),
- Spartha Medical (FR),
- Preste (FR),
- ANSES (FR),
- CSIC (ES),
- Fundacion Tekniker (ES),
- Steinbeis Advanced Risk Technologies Institute (RS),
- Dolmen Design and Innovation (IE),
- Dublin City University (IE),
- Aquamonitrix (IE),
- Karlsruher Institut fuer Technologie (DE),
- Kemijski Institut (SI),
- Betthera (CZ),
- EMPA (associated, CH),
- University of Nottingham (associated, UK),
- Preste Limited Liability Company (Affiliated Entity, UA)
Investment :
Total project EU grant: 7,15 millions euros CEA-Leti grant: 805 Keuros
Targeted Markets :
Chemicals and in-vitro toxicology
Keywords :
Toxicology, ecotoxicology, health assessment, risk assessment, Safe and Sustainable by Design (SSbD), in-vitro, in-silico, models
Credit photo :
CEA-Leti
|
There are major health and regulatory concerns about the potential toxicity of new chemicals and materials. In addition, safety assessment during the development of new chemicals still relies heavily on in vivo studies. As presented in the Commission initiative for Safe and Sustainable by Design (SSbD), the safety and sustainability assessment follows a step-wise approach, addressing hazard assessment, safety aspects of production and processing, human health, and eventually environmental sustainability assessment.
Currently, alternative test methods either do not exist or are not sufficiently predictive and can be prohibitively cost and time-consuming because of the high number of tests.
New methods, reducing animal testing and allowing a robust assessment of materials and chemicals that include the full sustainability and life cycle assessment are needed.
The challenge is therefore to reconcile the variety of testing methods, the lack of predictability, the need for high throughput testing, and expectations regarding interoperability and standardisation of methods by international regulatory bodies.
Objectives
- Safe and Sustainable by Design approach requires an entire life cycle monitoring of toxicity of chemicals.
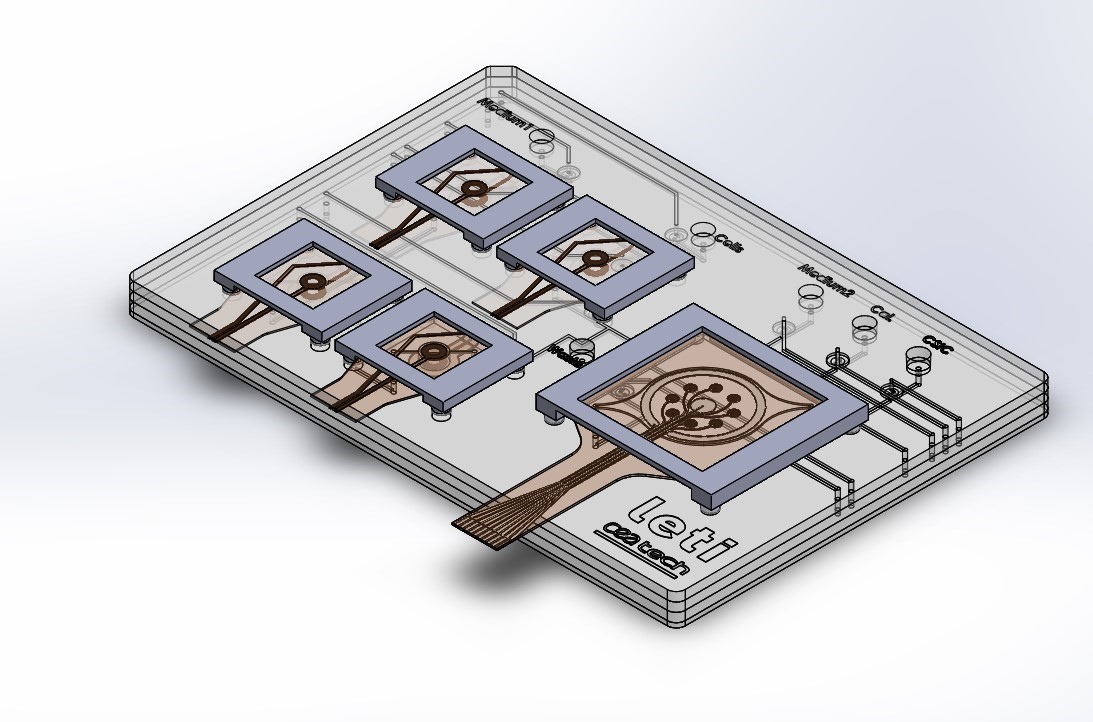
However, current testing systems cannot mimic the exposure conditions related to each step and are not compatible with downstream in silico analyses. New sets of instrumentation that enables modular testing capacities with integrated data bridging and progressive in silico model development systems are needed. TOXBOX will provide a device based on a prototype developed in a H2020 project in which CEA-Leti participated, PANBioRA, with a flexible microfluidic and instrument architecture to provide a plug and play testing platform to ease accessibility and interlaboratory validation.
The system will incorporate the following tests: automated cytotoxicity and genotoxicity tests, connected barrier/metabolic tissues coupled with cytokine and real-time electrochemical read-outs, and a testing module based on zebrafish embryo for ecotoxicology. The device will allow to study various life-cycle stages, i.e. raw materials at the production or degradation at the end-of-life, and to select the most relevant associated biological models, at single organ and systemic/full organism level, including human health and environmental assessment.
The system will be validated using metallic 2D structures and nanoparticles, biocides, and known endocrine disruptors. Custom made functional polypeptides (novel biocides) will be used to cover the design phase.
Progressive in silico models for long-term effects will be iteratively developed and used to predict each new chemical group to be tested until good predictability is achieved with new chemical formulations (comprehensive risk assessment). A data management platform that enables interfacing with the available databases will be developed. After interlaboratory validation of the device by 4 partners, a standardization folder will be prepared, to make the device available for testing at all stages of the material life cycle assessment to different stakeholders.
TOXBOX aspires to bring forth an instrument that will provide reliable toxicity data in relevant conditions for each chemical and enable reliable in silico model development.
|
|